Custom made EEG
Customized fit
Our 3D printed EEG technology allows physicians to easily apply and monitor patient brain activity patterns, ensuring good electrode contact at all times. Up to 34 electrodes can be placed freely according to any international EEG electrode system (10-20, 10-10, 10-5).
Repetitive assessments
Repetitive clinical assessments of brain activity becomes more easy. This allows monitoring of subtle brain changes over time, providing valuable insights into neurological conditions such as Alzheimer's disease.
EEG source imaging
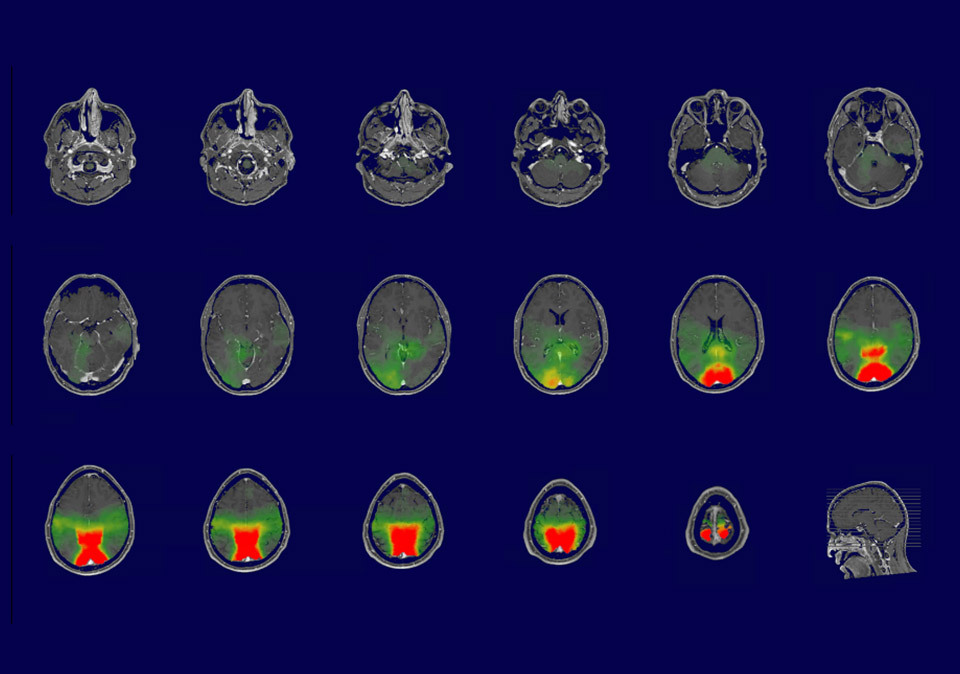
Our EEG system is combined with precise information of the head anatomy to allow EEG signals to be converted into neuroimaging modality using source localization algorithms.
Clinical grade EEG
Our custom made EEG device provides precise and continuous brainwave monitoring. Integrated into a personalized 3D printed cap, it allows long term remote tracking for clinical applications. By capturing repetitive data, it aids in identifying brain abnormalities, tracking changes, and discovering biomarkers for neurological conditions, such as Alzheimer's disease (AD), depression, PTSD, Parkinson's, epilepsy, improving understanding, diagnosis, and treatment.
The Bottneuro Custom tCS/EEG device is currently registered as a custom-made device (according to the UK MDR 2002 5 (1) & 15, Swiss MedDO Art. 10) for general tCS and EEG indications in the UK and Switzerland.
The prescribing qualified healthcare expert is responsible for the clinical application specific for an individual patient. The Bottneuro devices are currently not cleared for sales in the USA.